IIT Bombay breakthrough in CAR T-Cell and Adoptive T-Cell Therapies
- 09 Feb 2026
In News:
Researchers at Indian Institute of Technology Bombay (IIT Bombay) have addressed a key technical bottleneck in CAR T-cell and other Adoptive T-cell Transfer (ACT) therapies, safe recovery of lab-grown T-cells without loss of viability or immune function. The study demonstrates that using a gentler enzyme (Accutase) significantly improves therapeutic reliability and may reduce costs of cancer immunotherapy in India.
Basics for Prelims
T-Cells
- A type of white blood cell central to the immune response
- Detect and destroy infected or abnormal (cancerous) cells
- Coordinate other immune cells, making them crucial for immunotherapy
CAR T-Cell Therapy
- A personalised cancer treatment using a patient’s own T-cells
- Process:
- T-cells collected from patient’s blood
- Genetically engineered to express Chimeric Antigen Receptors (CARs)
- CARs act like GPS, guiding T-cells to cancer cells
- Cells are multiplied in the lab and infused back into the patient
- Approved globally for certain blood cancers (leukaemia, lymphoma)
- Indian milestone: NexCAR19, the world’s first humanised CAR-T therapy, developed by ImmunoACT
Key Research Development (IIT Bombay)
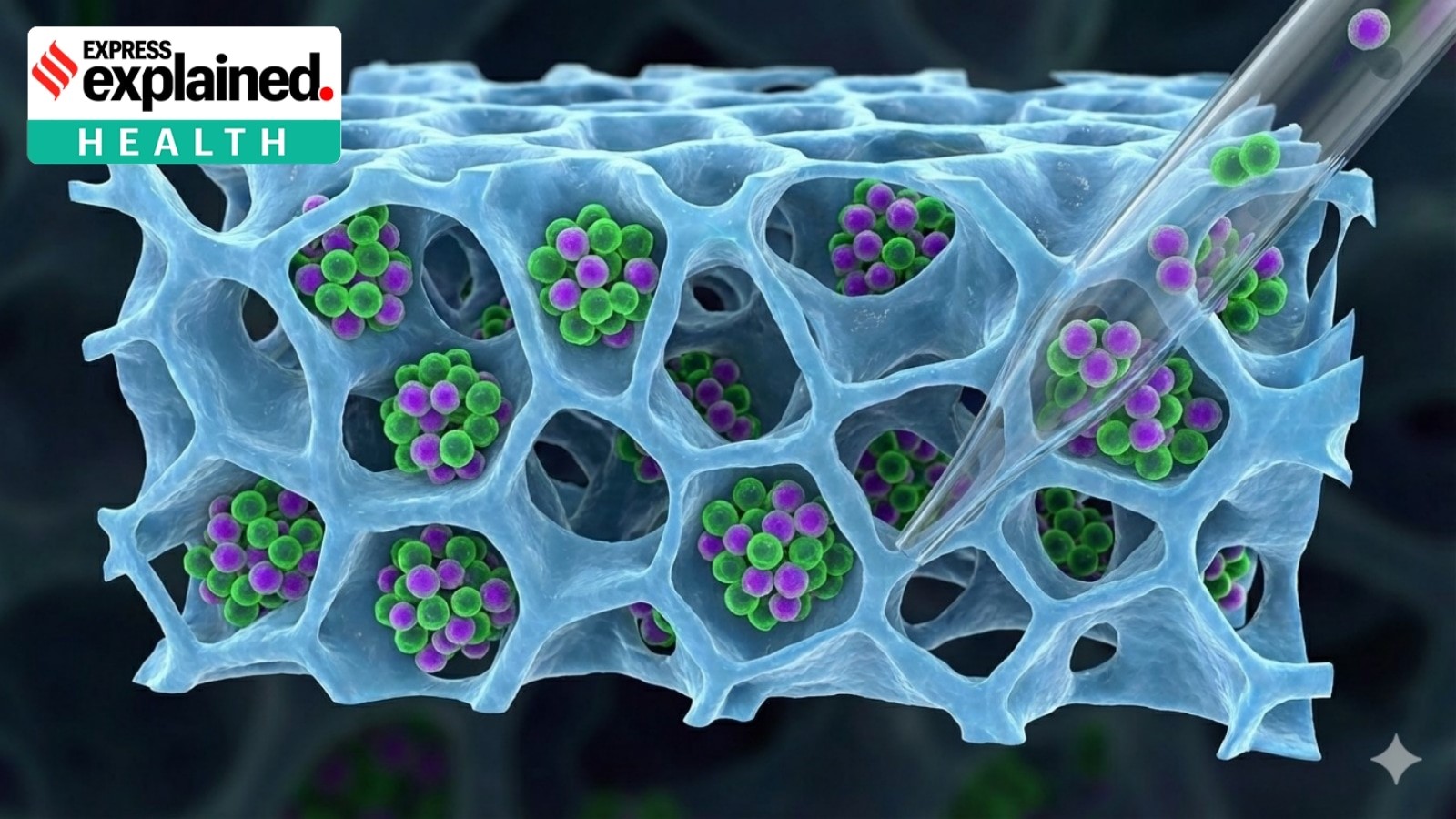
The Challenge
- T-cells are grown on 3D fibrous scaffolds to mimic the body’s environment
- These scaffolds improve growth and potency, but cells adhere tightly, making recovery difficult
- Harsh recovery methods damage surface proteins, reducing therapeutic effectiveness
Methods Tested
- Manual flushing
- TrypLE enzyme (harsh)
- Accutase enzyme (gentle)
Findings
- Cell yield: Similar across all methods
- Cell viability & immune function:
- TrypLE → higher cell death, reduced immune activity
- Accutase → preserved viability, clustering ability, and cancer-killing potency
- T-cells grown on scaffolds and recovered with Accutase remained highly effective against cancer cells
Significance of the Study
- Improves reliability of CAR T-cell and ACT therapies
- Potentially reduces production costs of immunotherapy
- Enhances India’s capacity for indigenous, affordable advanced cancer treatments
- Supports expansion of immunotherapy beyond elite centres
Benefits of CAR T-Cell Therapy
- Targeted precision: Spares healthy cells compared to chemotherapy
- Personalised: Uses patient’s own engineered cells
- Long-lasting protection: Engineered T-cells persist in the body
- Reduced hospitalisation and costs (especially with indigenous innovations)
- Expands future possibilities in cancer immunotherapy research
