P-47 Protein
- 12 Sep 2025
In News:
Proteins within living cells constantly face mechanical stress during processes such as intracellular transport, cytoskeletal remodeling, and degradation. These stresses can compromise protein folding and stability, leading to cellular dysfunction.
Traditionally, specialized proteins called canonical chaperones were considered the primary agents guiding protein folding and stability. However, recent research has uncovered a surprising player in this landscape—p47, a cofactor protein with previously underestimated roles.
Discovery by SNBNCBS
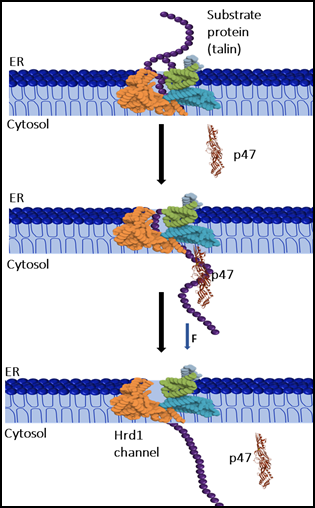
A study conducted by the S. N. Bose National Centre for Basic Sciences (SNBNCBS), an autonomous institute under the Department of Science & Technology (DST), revealed that p47 functions as a “mechanical chaperone.” Using single-molecule magnetic tweezers, researchers applied controlled mechanical forces to mimic stresses faced by proteins inside cells.
The experiments demonstrated that:
- p47 stabilizes mechanically stretched proteins, enabling them to refold even under constant pulling forces.
- It enhances the mechanical efficiency of protein extraction from the endoplasmic reticulum (ER) lumen to the cytoplasm.
- It facilitates polypeptide translocation through narrow pores, reducing misfolding risks and improving protein quality control.
About p47 Protein
- Nature: A cofactor protein traditionally associated with the p97 complex, a major cellular machine responsible for protein trafficking, degradation, and membrane fusion.
- Revised Role: Beyond being a passive assistant, p47 exhibits autonomous, force-dependent protective activity, extending the functional repertoire of accessory proteins.
Significance of the Findings
- Scientific Breakthrough
- This is the first direct, single-molecule evidence that cofactors can act as mechanical chaperones.
- It challenges the existing view of accessory proteins as mere helpers and redefines their role in protein mechanics.
- Therapeutic Potential
- Targeting p47 or similar cofactors may provide new treatment avenues for diseases where protein stability under stress is compromised, such as:
- Cardiomyopathies (heart muscle diseases).
- Laminopathies (genetic disorders linked to nuclear protein instability).
- This could lead to precision medicine strategies focusing on mechanical resilience of proteins.
- Targeting p47 or similar cofactors may provide new treatment avenues for diseases where protein stability under stress is compromised, such as:
- Broader Implications
- Enhances understanding of cellular stress response mechanisms.
- Opens possibilities for drug development aimed at modulating protein folding under force.
- Strengthens India’s contributions to cutting-edge biophysical research with global relevance.
